
It has been about five months since our last article on COVID-19. Trusted sources of mainstream media started providing good coverage at that time, so we decided to focus on articles about each of the 20 Key Health Factors for 2020. Those are now complete.
Much more importantly with the recent resurgence of COVID-19 in Western Europe and expected new wave of infections in the United States this fall and winter, it is time for increased vigilance on your personal safety and that of your friends, family and co-workers
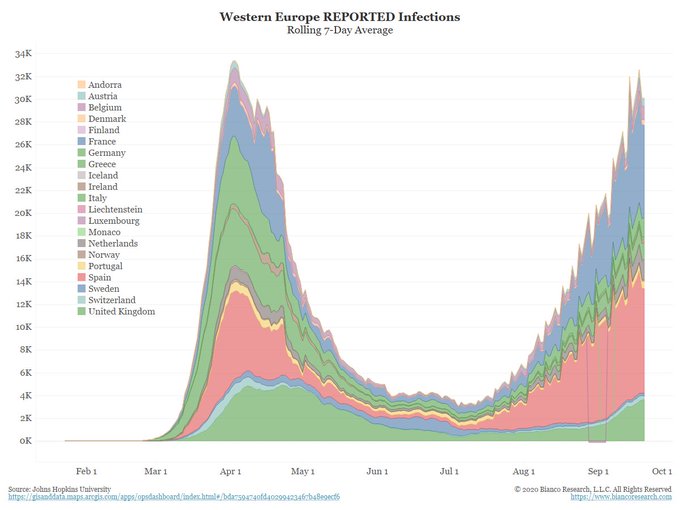
COVID-19 continues to spread around our world rapidly.
As of September 28, 2020, confirmed worldwide cases increased to over 33 million, up from 3 million just five months ago. ~24 million people have recovered. ~1 million people have died. The actual number of worldwide cases (and deaths) is higher as many infected people were not tested.
According to a Financial Times study, the 2020 death rate in some countries is 50% higher than usual all-cause mortality rates. In many countries, these excess deaths exceed reported numbers of Covid-19 deaths by large margins.
The COVID Symptom Study, conducted by leading medical institutions in the United States and Europe, has found that 10-15% of the infected have lingering and lifelong serious impairments, even those who had mild symptoms.
Ongoing problems include fatigue, a racing heartbeat, shortness of breath, achy joints, foggy thinking, a persistent loss of sense of smell (and taste) and serious damage to the heart, lungs, kidneys, and brain.
Warm-weather months have allowed for masked, socially-distanced outdoor activities together with friends and family. These are low-risk activities provided people actually remember to keep their masks on and avoid yelling, cheering, singing and other loud vocal activities which spray germs. Washing your hands before and after is still important, too.
Now, with summer over and colder weather coming, there is valid concern for a new wave of infections as people move back indoors, less cautious than they should be. Indoor air-conditioning and heating dry out our noses which filter out viruses. This leaves us more susceptible to influenza, common coronaviruses (the common cold) and COVID-19. In addition to protecting others from us, masks increase warmth and humidity in the air we breathe, providing us increased protection through our nasal mucosa.
COVID-19 in 2021
Mid-2021 is when government and other medical experts predict that COVID-19 vaccines will become widely available. Although this is good news, Pew Research’s September 2020 study of 10,000 Americans found that only 51% are definitely or probably willing to get vaccinated, of which less than 25% were in the definitely category. Until more clinical trial data comes in, many people have justifiable concerns about safety and efficacy.
Two-thirds of the population would need to get on board with vaccination in order to achieve herd immunity with a 75% effective vaccine.
Leading COVID-19 Vaccine Candidates
The US Government and the Center for Epidemic Preparedness (CEPI) have funded six leading vaccine candidates with $1.5-2.5B each. That sort of funding gives these corporations a large advantage over their competition. Here’s a quick status update on each of them. They are in order of how soon they might be available.
Pfizer / BioNTech; Their second candidate mRNA two-dose vaccine has generated neutralizing antibodies similar to those from infected patients. The average level of antibodies generated in older adults was only 41% of that seen in younger participants. However, it was still higher than the level of antibodies seen in recovered patients. They’ve received good results in limiting common vaccine side-effects.
Their 30,000 patient global Phase 3 double-blind clinical trial in healthy volunteers from ages 18 to 85 started July 23, 2020 with results expected in late October / early November. Half the volunteers will receive a placebo which makes it a bit strange to participate since you will still need to avoid risk of infection! And if their volunteers all avoid COVID-19, then how do they know if the vaccine worked?
Also, distribution will be a challenge for Pfizer/BioNTech because they require -94 degree Fahrenheit cold storage freezers. Most hospitals, pharmacies and clinics as well as drug transportation firms do not have this infrastructure in place.
Moderna — They started a 30,000 patient global Phase 3 double-blind trial on July 23rd with results expected in Q4, 2020 after the election. Moderna is gearing up to produce 500 million to 1 billion doses of the potential two-dose mRNA vaccine annually. The vaccine needs to be kept at normal freezer temperature (-4 degrees Fahrenheit) in distribution centers, and at normal refrigerator temperature (36 to 46 degrees Fahrenheit) at point-of-care sites.
Astrazeneca / Oxford; Their two-dose adenovirus 5 vaccine is similar to the leading Chinese (Sinovac, Sinocure) and Russian (Gamalaya Research Institute) vaccines which use inactivated virus. These vaccines have shown the ability to generate neutralizing antibodies in all participants as well as T cell responses. However, two rare (1 in 250K) disease indications of spinal cord inflammation occurred in 1400 healthy volunteers in the Astra clinical trial. Experts were already wary of side-effects from prior attempts at adenovirus vaccines. Mike Roizen, the CMO of The Cleveland Clinic does not recommend participating in adeno clinical trials. The trial is currently paused by the National Institute of Health within the United States, but continues elsewhere.
Johnson & Johnson / Janssen — They initiated a 60,000 patient global Phase 3 trial in September, 2020, covering patients in high-risk places. The single-dose Janssen COVID-19 vaccine candidate leverages their AdVac® technology platform, which was also used to develop and manufacture Janssen’s European Commission approved Ebola vaccine and construct its Zika, RSV, and HIV vaccine candidates. AdVac® has been used to vaccinate more than 100,000 people to date across Janssen’s investigational vaccine programs.
With Janssen’s AdVac® technology, the vaccine, if successful, is estimated at launch to remain stable for two years in normal freezers and at least three months in refrigerators. This makes the vaccine candidate compatible with standard vaccine distribution channels and would not require new infrastructure to get it to the people who need it.
Novavax — They initiated a 10,000 patient UK Phase 3 trial in September, 2020 and are running Phase 2 trials in the United States, Australia and South Africa. Novavax’s vaccine is a stable, prefusion protein made using proprietary recombinant protein nanoparticle technology including MatrixM™ adjuvant. The vaccine is an unfrozen, liquid formulation that can be stored in refrigerators, allowing for distribution using standard vaccine channels.
Novavax has continued to scale-up its manufacturing capacity, currently at up to 2 billion annualized doses, once all capacity has been brought online by mid-2021.
Sanofi / Glaxo-Smith Kline (GSK) — Sanofi uses its S-protein Covid-19 antigen in a vaccine candidate with a GSK adjuvant. Based on preclinical data showing an acceptable tolerability profile and the production of neutralizing antibodies at levels comparable to those seen in convalescent patients, they launched a Phase I/II trial in September, 2020. They are seen as trailing other solutions, but have deals with the EU for 300 million doses and the US for 100-600 million doses.
For those who would like to know more about other vaccines, EndPoints News has ranked the top 29 candidates. For access, you must subscribe to their free mailing list.
The Big Unknown COVID-19 Questions
- What will be the duration of immunity provided by COVID-19 antibodies? For common coronavirus infections, the period of antibody resistance can be only 2-3 months. Medical experts are expecting that COVID-19 will require annual vaccinations. This is why it will be so lucrative for the companies who succeed in commercializing their vaccines!
- Are T-Cell responses required for quality and durability of immunity? Many of the leading vaccines are focused solely on a single protein, called the spike protein, as the immunizing antigen.
- What unexpected negative side-effects will come from the large clinical trials?
- If only 50% of people will take a vaccine, how does that impact returning back to normal life? Can only vaccinated kids go to in-person school? Can only vaccinated adults show up for work?
So, what next for you? Stay safe until you can get a vaccine which has been sufficiently tested.
- Hope that you can get vaccinated safely by the middle of 2021.
- Until then, don’t isolate yourself. There are many health benefits of social interaction. However, limit social interaction and communications with co-workers, friends and family to socially distanced, masked outdoor settings or through voice and video calls.
- Share this article with co-workers, friends and family as a reminder for them to be careful. If they stay safe, that helps you stay safe.
Thanks for reading. As your reward for continuing to focus on your health, go get a flu shot. Wait, what? That doesn’t sound like much of a reward? Okay, so treat yourself to something nice at the pharmacy or after your visit your doctor. 🙂 Getting your flu shot is extra important this year. Even in normal times, high risk patients over 50 who get flu shots are 28% less likely to die the next year from cardiovascular events and have a 73% lower risk of death.
Still don’t think this is a good enough reward for reading? Okay, then go figure out how to decorate your front door for Halloween with these creative ideas.
